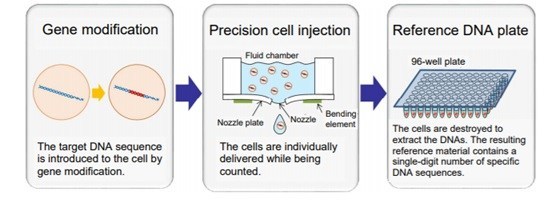
Researchers affiliated with the Kawasaki INnovation Gateway at SKYFRONT have used bioprinting technology to produce new reference DNA material in which the absolute number of the DNA molecules is controlled in units of one. The reference material can be used in the quality control of genetic testers and reagents.
(Photo: https://mma.prnewswire.com/media/745971/KAWASAKI_CITY_Bioprinting.jpg )
Further information about science and technology projects at Kawasaki City is available in the Kawasaki SkyFront iNewsletter that highlights research being conducted by scientists and industries affiliated with Kawasaki INnovation Gateway at SKYFRONT (KING SKYFRONT)-the City’s flagship science and technology hub launched in 2013 to focus on open innovation in the life sciences and environment.
September 2018 issue of Kawasaki SkyFront iNewsletter
http://inewsletter-king-skyfront.jp/en/
Research highlight
http://inewsletter-king-skyfront.jp/en/research_highlights/vol-13-research01/
Bioprinting technology to control the number of DNA Molecules to single molecule level
Contributing to high accuracy gene testing using reference DNA material containing well defined number of DNA molecules
The polymerase chain reaction (PCR) is a high sensitivity method that is extensively for genetic testing with reports showing the detection of single DNA molecules by amplification. Specific applications include inspections of genetically modified organism foods, cancers, and infections. As part of such tests, it is important not to miss a specific DNA sequence (target gene) to be examined, and it is important that inspection agencies control of the quality of inspection instruments, reagents, and detection methods as a whole.
Some companies and research institutes have delivered reference material whose DNA types and densities are prescribed, but they are of high densities i.e. the number of DNA molecules is prescribed in mols (one mol is equivalent to 6.02×1023 DNA molecules). For use in low-density tests at an accuracy of 100 molecules or less, the reference material generally must be diluted. Thus, errors can occur in DNA molecule densities during the diluting process-the resulting samples may contain more DNA molecules than prescribed, or conversely no DNA molecules at all, when the required number of DNA molecules is less than ten.
With this background, Ricoh Company, Ltd., the National Agriculture and Food Research Organization (NARO), and FASMAC of the Nippon Flour Mills Group have collaborated to use bioprinting technology to produce new reference DNA material in which the absolute number of the DNA molecules is controlled in units of one. The reference material can be used in the quality control of genetic testers and reagents.
The three organizations developed a new method to produce reference DNA material, which enables production of reference materials suitable for genetic tests to detect specific DNAs, as in the inspection of GMO foods, cancers, and infections. The new reference DNA materials will increase the reliability of the tests.
Reference
Presented at the Bio International Convention in Boston, USA (June 4-7th, 2018) and Biotech in Tokyo, Japan (June 27-29th 2018).
About KING SKYFRONT
KING SKYFRONT is located on the opposite side of the Tama River that separates Tokyo International Airport (also known as Haneda Airport) and the Tonomachi district of Kawasaki. The Airport plays an important role in the globalization of the innovative activities of scholars, industrialists and City administrators based at KING SKYFRONT.
KING SKYFRONT was launched in 2013 as a base for scholars, industrialists and government administrators to work together to devise real life solutions to global issues in the life sciences and environment.
Further information
Kawasaki City, Japan, Coastal Area International Strategy Office
General Planning Bureau, City of Kawasaki,
1 Miyamoto-cho, Kawasaki-ku, Kawasaki-city, Kanagawa 210-8577 Japan
EMAIL: 20rinkai@city.kawasaki.jp
SOURCE KING SKYFRONT