COVID-19 Diagnostics Market Size Worth $283.3 Billion By 2028 | CAGR: 13.6%
The global COVID-19 diagnostics market size is expected to reach USD 283.3 billion by 2028 according to a new study by Polaris Market Research. The report “COVID-19 Diagnostics Market Share, Size, Trends, Industry Analysis Report, By Product & Service Type (Instruments, Reagents & Kits, Service); By Sample Type (Nasopharyngeal (NP) Swab, Oropharyngeal (OP) Swab, Nasal Swabs, Blood, Others); By Test Type (Molecular (PCR) Testing, Antigen-based Testing, Antibody (Serology) Testing, Others); By Mode; By End Use; By Regions; Segment Forecast, 2021 – 2028” gives a detailed insight into current market dynamics and provides analysis on future market growth.
COVID-19 medical tests play an important role in management of coronavirus and thus helping in containing the virus infection. These tests are available as POC and non-POC across various healthcare settings such as hospitals, diagnostic centers, physician clinics, and research laboratories. POC test are comparatively easier than the other swab-based tests but they lack sensitivity and specificity thus providing a non-conclusive result.
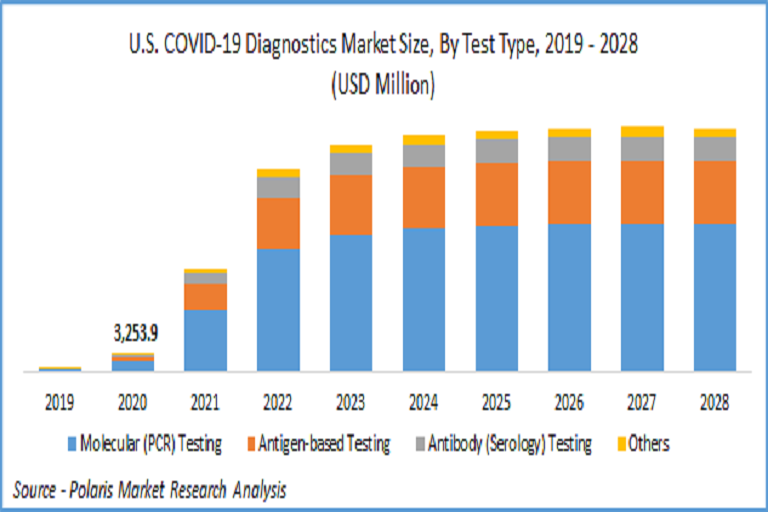
While these tests are useful in real-time monitoring of infection as a preliminary test, the swab-based tests are necessity for confirmation of COVID-19 infection as they incorporate RT-PCR technology, which provide accurate results. The recent requirements for COVID-19 tests are higher accuracy, faster results, low-cost, and high sensitivity towards coronavirus. The market for PCR technology was estimated to be 69.5 billion in 2021 and is expected to grow at a CAGR of more than 13% during the forecast period.
The market drivers for COVID-19 diagnostic includes rapidly increasing COVID-19 infections across the globe, increasing number of patients undergoing diagnosis, government initiatives such as Sero testing, increased preference of physicians and patients towards POC testing, and increased requirement for COVID-19 test by non-profit organizations.
According to World Health Organization (WHO) as of 18 June 2021 there were 177,108,695 confirmed cases of COVID-19 infections worldwide, affecting about 213 countries and increasing rapidly. The relaxation in lockdowns across the countries and increasing number of testing is expected to drive the demand for COVID-19 diagnostics market. In addition, emergence of third wave of infections in Europe is likely to have a positive impact on the market growth. The European market is expected to grow at a CAGR of 13.7% from 2021 till 2028 which is followed by North America. However, Asia Pacific region is expected to remain the largest market.
View more Information About COVID-19 Diagnostics Market @ https://www.polarismarketresearch.com/industry-analysis/covid-19-diagnostics-market/request-for-sample
Companies such as Cepheid, 1drop Inc., ALDATU BIOSCIENCES, Altona Diagnostics GmbH, Perkin Elmer, Inc., bioMerieux SA, Danaher, Hologic Inc., Abbott, Luminex Corporation, ADT Biotech, Mylab Discovery Solutions Pvt Ltd., Laboratory Corporation of America Holdings, Quest Diagnostics, Quidel, Neuberg Diagnostics, Thermo Fisher Scientific, Inc., F. Hoffman-La Roche Ltd. and Veredus Laboratories are some of the key players operating in the market.
Key market participants are currently focusing on manufacturing and distribution of diagnostic test kits and service to cater to ever increasing demand in pandemic across the globe. In March 2020, Roche shipped its first set of diagnostic kits namely Cobas SARS-CoV-2, of which about 400,000 kits were shipped to in-network distributors across the U.S. ensuring faster reach to the end-users.
Additionally, some biotech companies are launching new PoC diagnostic tests, for instance, E25Bio, a U.S.-based Biotech firm has developed a new test which provides conclusive results within 15 minutes. In addition, Germany-based diagnostic equipment manufacturer Bosch has developed a rapid testing kit for COVID-19 which claims to detect virus within two and a half hours, such initiatives and product launch are expected to increase over the coming years.
Polaris Market Research has segmented the Covid-19 diagnostics market report on the basis of product & service, sample type, test type, mode, & end-use
COVID-19 Diagnostics Product & Service Outlook (Revenue – USD Million, 2019 – 2028)
Instruments
Reagents & Kits
Service
COVID-19 Diagnostics Sample Type Outlook (Revenue – USD Million, 2019 – 2028)
Nasopharyngeal (NP) swab
Oropharyngeal (OP) swab
Nasal Swabs
Blood
Others
COVID-19 Diagnostics Test Type Outlook (Revenue – USD Million, 2019 – 2028)
Molecular (PCR) Testing
Antigen-based Testing
Antibody (Serology) Testing
Others
COVID-19 Diagnostics Mode Outlook (Revenue – USD Million, 2019 – 2028)
Point-of-Care (PoC)
Non Point-of-Care (Non-PoC)
COVID-19 Diagnostics End-Use Outlook (Revenue – USD Million, 2019 – 2028)
- Laboratories
- Hospitals
- Diagnostic Centers and Clinics
- Research Institutes
COVID-19 Diagnostics Regional Outlook (Revenue – USD Million, 2019 – 2028)
North America
U.S.
Canada
Europe
France
Germany
UK
Italy
Spain
Netherlands
Russia
Asia Pacific
China
India
Japan
Malaysia
South Korea
Indonesia
Central & South America
Mexico
Brazil
Argentina
Middle East & Africa
Iran
Saudi Arabia
Iraq
South Africa
About Us
Polaris Market Research is a global market research and consulting company. The company specializes in providing exceptional market intelligence and in-depth business research services for our clientele spread across different enterprises. We at Polaris are obliged to serve our diverse customer base present across the industries of healthcare, technology, semiconductor.