How would complete end-to-end workflows and collaboration tools enable us to have efficiency in our systems? Economies of scale within a business are often difficult to achieve as more and more businesses convert their legacy studies. Quality, delivery and functionality issues although they exist reduce business needs. Data integration-as-a-service or DIAAS is slowly emerging as a one stop shop for data transformation that permeates any industry or proprietary target standards. Will conversions to following formats such as CDISC standards like ODM, CDASH, SDTM, LAB, ADaM or even implementing BRIDG model. We discuss whether these systems have significant business value through increased automation of key portions across legacy clinical data conversion processes that use Business Process Management tools.
What is the optimal Infrastructure cloud?
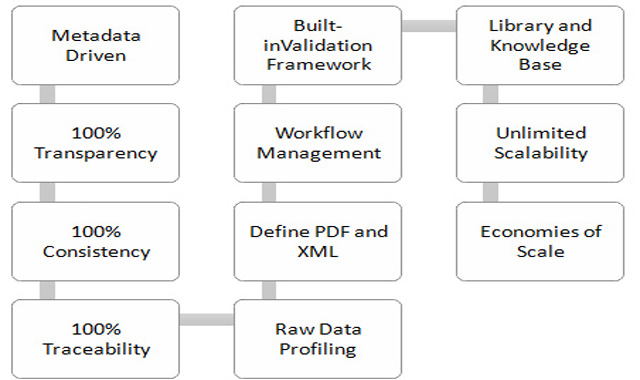
If you are looking for a system that offer unlimited scalability and complete security for your clients then look for expert technological consultants. Legacy studies and ensuring live studies with Data Warehousing and Regulatory submission can use this system. Overall one needs to implement DIaaS across lifecycle stages should ensure that the platform has the following:
Overall, the infrastructure platform should have definite factors towards its approach. Having a metadata driven approach ensures that the company has significant control over the legacy study data conversions. Thereby, ensuring its success and reaping the benefits provided. So, what are the challenges faced by a regular company that is looking towards implementing legacy study data conversions:
- Adapting to the constant changing requirements
- Dealing with the Enterprise view which has always been a mirage
- Always dealing with a slow turn around
- Dealing with organic growth
What are the best solutions that technological major such as HCL Tech offer to meet these challenges:
- One can govern and manage requests as well as enhance knowledge
- Develop significant enterprise level semantics
- Improve analysis, design and ensure development
- Ensure the adoption of application and agnostic data architecture
One of the critical ways in which one can approach these solutions is by providing metadata driven approach that overall manages intelligence as well automates the mechanics involved.
Be it meeting your business benefits or optimising production, the DIaaS platform ensures cost reduction in sponsor with simplified pricing and significant error reduction. One also ensures control with the standardized and driven approach by eliminating the “black box processes”, eliminating bottle necks and ensuring process visibility for sponsors. With enhanced communication, single version of truth, ensuring reduction in human error and built-in quality control frameworks, quality issues are taken care of. Performance errors can be reduced by streamlining processes and automating programming schedules.
To know more about the topic please visit HCL Technologies.
Source : HCL Technologies