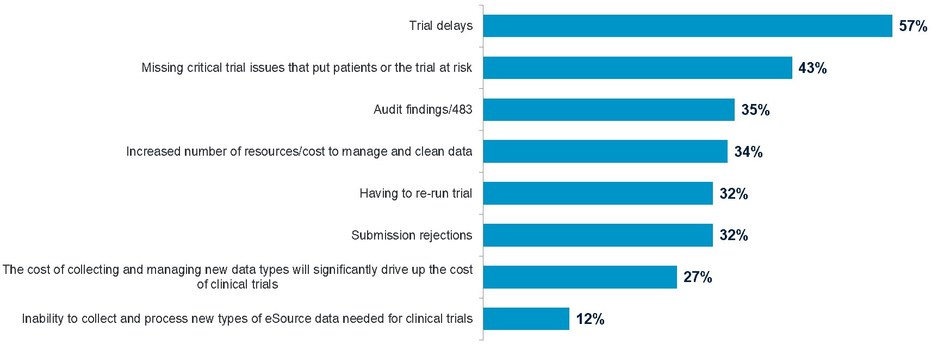
Pharmaceutical companies share significant concerns over their ability to bring more drugs to market faster due to clinical data challenges. A new global study, commissioned by Oracle Health Sciences and conducted by Pharma Intelligence, revealed that 57 percent of the clinical researchers surveyed believe that their clinical data issues result in trial delays.
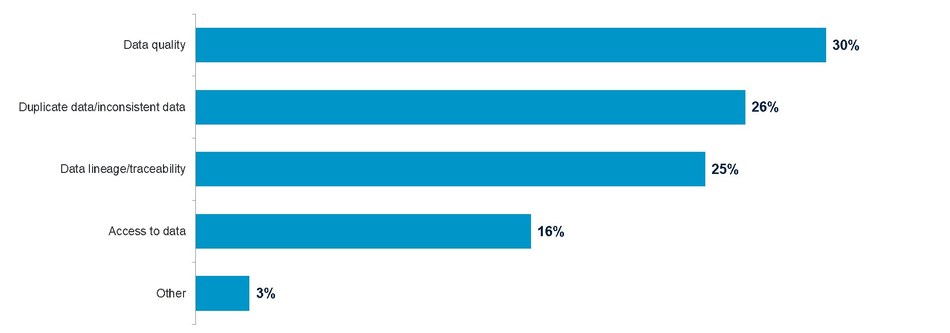
In addition to trial delays, 81 percent of respondents cited data governance issues as the biggest challenge in meeting regulatory compliance. The top three data issues according to survey respondents were duplicate data/inconsistent data, data quality and data integrity/traceability.
“Data governance is our top concern because clinical data quality issues can hinder a trial’s completion,” said Melonie Longan, Director, Data Operations, Functional Services, Premier Research. Premier Research is a CRO serving biotechnology customers who are researching therapies related to analgesia, dermatology, hematology, oncology, neuroscience, pediatrics, and rare disease.
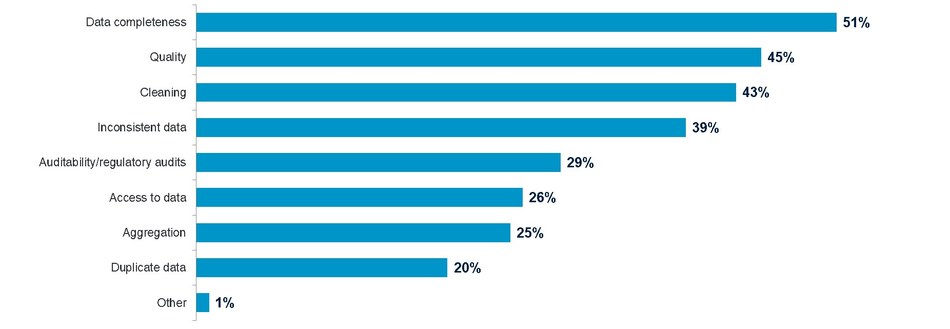
When asked what the top three operational challenges were with their clinical trial data, 51 percent cited data completeness, 45 percent said data quality and 43 percent, data cleaning.
“The kind of clinical data quality issues such as those highlighted in this report can have significant negative impacts,” said Julie Barenholtz, Principal Clinical Data Manager, Cytel Inc. “As a data company, we are always looking for ways to improve the quality of the data and process it efficiently so that patients have access to treatments as quickly as possible.” Founded in 1987, Cytel is a multinational contract research organization (CRO) known for its pioneering work in statistical science and the design and implementation of adaptive clinical trials.
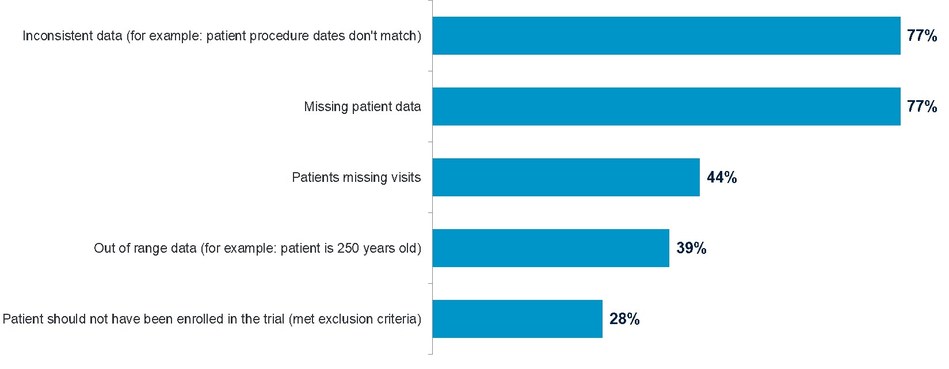
Not surprisingly, over three-fourths of respondents cited inconsistent data and missing patient data as the most critical clinical data problems to catch in clinical trials.
“Clinical teams are forced to spend time cleaning data instead of analyzing it, and they can’t always see the entire picture of what is available to them, this delays the ability to make critical decisions about the trial and holds up regulatory submission,” said Steve Rosenberg, General Manager, Oracle Health Sciences. “Clinical researchers shouldn’t have to spend time and resources on fixing data issues that technology was built to handle. Technology can, and should, be used to eliminate unnecessary manual intervention and mitigate risk so we can get therapies in the hands of patients who are waiting.”
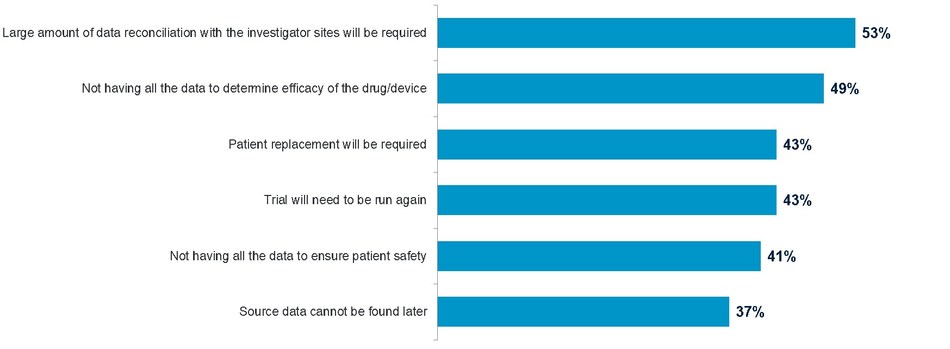
The top three risks highlighted by the research include the need for additional data reconciliation; incomplete data to determine efficacy and patient replacements.
“Wasting precious time reconciling clinical data issues can be detrimental and costly to our customers,” said Vicki Gashwiler, Associate Director, Strategic Development & Market Access, MedTech Division for Novella Clinical. “Our top concern is proactive data management and data monitoring to reduce the risk of clinical data quality issues slowing down a trial. These delays can have significant financial implications for our customers.”
About the Survey
The survey was conducted by Pharma Intelligence and sponsored by Oracle Health Sciences and ran from the beginning of August 2018 into September 2018. The largest percentage of responses came from clinical researchers, data scientists and clinical programmers from biopharma organizations and a small percentage of medical device companies and Contract Research Organizations. Respondents were from around the globe with 61 percent from North America, 20 percent from Asia Pacific and 17 percent from Europe.
Additional Resources
- Download the full research report
- Learn more about Data Management Workbench
- Join the live webcast featuring experts from Oracle Health Sciences and Pharma Intelligence to understand how to prevent data quality issues from interrupting clinical trial progress on October 3, 2018 at 11 am EDT
About Oracle
The Oracle Cloud offers complete SaaS application suites for ERP, HCM and CX, plus best-in-class database Platform as a Service (PaaS) and Infrastructure as a Service (IaaS) from data centers throughout the Americas, Europe and Asia. For more information about Oracle (NYSE :ORCL ), please visit us at oracle.com
About Oracle Health Sciences
Oracle Health Sciences breaks down barriers and opens new pathways to unify people and processes to bring new drugs to market faster. As the number one vendor in Life Sciences (IDC, 2017), the number one provider of eClinical solutions (Everest Group, 2017) and powered by the number one data management technology in the world (Gartner, 2018), Oracle Health Sciences technology is trusted by 29 of the top 30 pharma, 10 of the top 10 biotech and 10 of the top 10 CROs for clinical trial and safety management around the globe.
Trademarks
Oracle and Java are registered trademarks of Oracle and/or its affiliates. Other names may be trademarks of their respective owners.
SOURCE Oracle